Blog - Keeping up with innovation
27 June 2024
By Huw Owen, Nomenclature Developer at the GMDN Agency
The MedTech industry is rapidly changing, with new developments regularly replacing existing technology. As a medical device nomenclature, the Global Medical Device Nomenclature (GMDN) helps to categorize, sort, and track the everchanging landscape of the medical device industry. In this blog, I’m going to tell you a bit about how we manage our Database to capture innovation and keep up with the hard work of researchers and manufacturers.
If a new device can’t be accurately described by an existing GMDN Term, then either an existing Term must be expanded or a new Term must be created. Does this mean that each new and expanded Term represents an innovation? The short answer is no. New Terms and expansions for non-innovative technologies may be required for a few reasons. The device may be an existing technology, not previously regulated as a medical device (e.g., furniture or athletic training equipment). Alternatively, the device may require a new Term to represent a variation of an existing technology. Examples of this include single-use/reusable counterparts and devices where clinically relevant features, such as antimicrobial properties, have been added.
So, what is an innovation? Two of the Regulators leading the charge on defining innovation are the Food and Drug Administration (FDA) in the US and the Medicines and Healthcare products Regulatory Agency (MHRA) in the UK. Both have different definitions of an innovative device, but it does seem there is a common understanding that innovation presents in different ways. Below is a diagram displaying the relationship between the two Regulators different methods of defining an innovation.
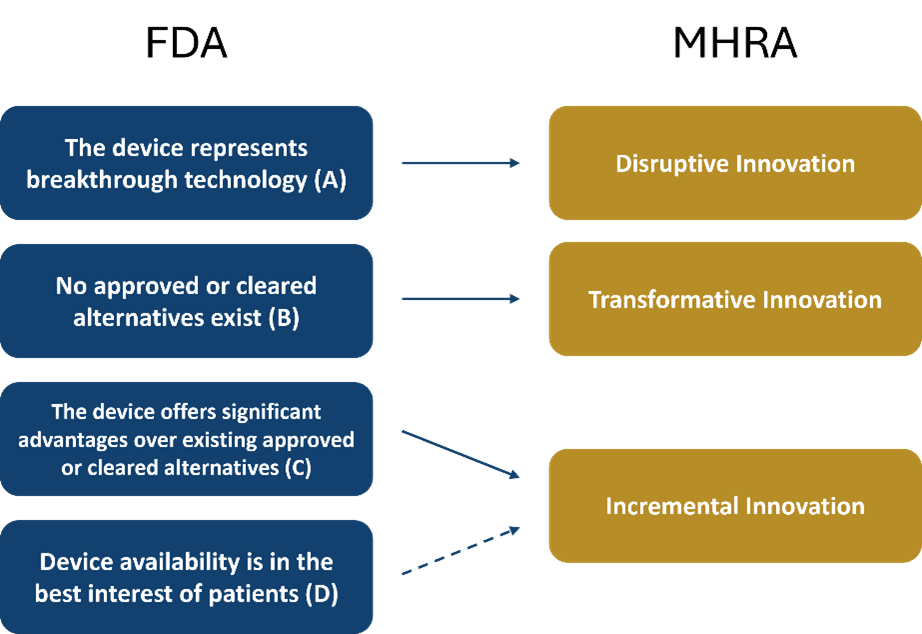
The FDA refer to innovative devices as Breakthrough Devices and have a range of criteria, two of which must be met for a device to be considered as such. The first must be met by all hopeful innovations and states ‘The device provides for more effective treatment or diagnosis of life-threatening or irreversibly debilitating human disease or conditions. There are then four options for the second criterion, of which one must be met. In the interest of future reference, I am going to refer to these as FDA A, B, C, and D and they are as follows: The device represents breakthrough technology (A), no approved or cleared alternatives exist (B), the device offers significant advantages over existing approved or cleared alternatives (C), or finally, device availability is in the best interest of patients (D).
Like the FDA, the MHRA has opted for a multi-class approach with devices falling into three separate classes: Incremental, Transformative or Disruptive innovation. Incremental innovation is defined as ‘an improvement to a device that already exists within the health system that positively affects the delivery of care or wider system’. Transformative innovation is defined as ‘an existing technology that is applied to the healthcare system for the first time or the application of a technology which is already in the healthcare system in a novel way for example, an existing device being applied to a new speciality’. The third class of innovative device according to MHRA definitions is Disruptive innovation. which is completely novel, with a foundational technology which doesn’t exist elsewhere, in or outside of the healthcare sector.
Despite the different approaches, there is some alignment between these concepts. The devices most likely to create headlines are FDA A and MHRA Disruptive innovations as these definitions describe a truly novel device. Within the GMDN, these devices will always require a new GMDN Term as, by definition, no existing device will utilise the same or similar technology.
Whilst not an exact match, FDA B most closely aligns to the MHRA’s Transformative innovation definition. These can be existing devices seeking clearance for a new clinical application and will typically trigger an expansion to an existing GMDN Term to add an additional use case to a device with the same technology and other attributes.
FDA C and the MHRA’s Incremental innovations represent the most common form of innovation, an existing technology which has been improved upon to provide better outcomes for the same intended use. As technology and intended use are two of the key attributes used to group devices into GMDN Terms, this type of innovation may not necessarily warrant a new Term, rather it may trigger an expansion to an existing Term. For example, the GMDN Term, Custom-made talus prosthesis, was recently expanded to include a custom-made talus prosthesis which is also transferable to other parts of the body (e.g., the carpals, cuneiforms, and femoral condyles), as a result the Term was renamed Custom-made hand/foot bone prosthesis. In some cases, the expansion required to include these innovations can blur the boundaries between two or more Terms. Because GMDN Terms are written to be mutually exclusive, and narrowing the scope of Terms is not allowed because it would result in the exclusion of previously assigned devices, the only option we have in this situation is to make one or more Terms obsolete.
The remaining definition – FDA D – doesn’t specifically align with any of the MHRA’s definitions. In some cases, FDA D is likely to refer to the production and distribution processes which improve the availability of the devices; in such cases this innovation is unlikely to be captured by the GMDN. However, technological advancements can provide greater availability and accessibility, in which case FDA D begins to overlap with the MHRA’s Incremental innovation definition. An example of this is portable versions of existing technologies, which can improve access to patients unable to make it to a clinical setting and home-use devices intended to be operated by a patient or other lay person which can help to account for shortages of clinical professionals. These are both concepts by which GMDN Terms are differentiated and the creation of either a portable or home-use version of a device will always be captured within the GMDN with the creation of a new GMDN Term.
As a medical device nomenclature, global harmonisation of the language surrounding the medical device industry is at the heart of what we do. It is for that reason the GMDN are currently working on new resources aimed at facilitating the analysis of innovation across the medical device industry.
