Guest Blog - The GMDN in a Swedish context

8 January 2025
Guest Blog – The GMDN in a Swedish context
By Annika Billberg, MTPReg Group
The Global Medical Device Nomenclature (GMDN) was introduced in Sweden in the year 2011. This was the year the MTPReg group was formed and started their assigned task of setting codes for all medical equipment owned by the Swedish public healthcare providers, the public hospitals.
In Sweden, the healthcare system is decentralized, which means that it is managed and run either by the regions, local authorities, or municipality. Health, medical, and dental care is divided into public and private sectors.
Currently, Sweden is divided into 21 regions. Each region has its own medical equipment register. The codes for the medical equipment in the local registers are all set by the MTPReg group. It is not possible to create any codes based on the GMDN code locally. Every night the MTPReg register synchronizes with all 21 regional registers so that all new codes set the day before instantly become available in the local registers.
In 2007 representatives for all 21 Swedish regions agreed to create a national code system, called MTPReg. It had become obvious to the regions’ medical device experts that it was impossible to compare medical equipment between regions, or to make a national inventory of all equipment in Sweden’s approximately 80 hospitals, unless you knew that you were addressing the same equipment. Hence the idea of a national code system was born and the GMDN was chosen to be a part of it.
Today we are six people working in the MTPReg group. We work part-time with the Swedish coding system; all six of us have other work to do besides the MTPReg coding. We are all employed as Medical Engineers in hospitals run by the Swedish regions.
We receive about 1,000 requests for new codes yearly. A customer suggests a code, which they find fitting for a medical device. The MTPReg code carries information about the term, the producer and the model of the medical device. We then compare the supplied information with the request to either accept or deny the suggested code. Including two photos for a new code is a prerequisite for a code request to be accepted. One photo shows the instrument while the other is a photo of the instrument label. During synchronization these photos are downloaded to each local database, together with the code information.
We download the GMDN Term and Term description in English to our MTPReg register. The term is translated to Swedish while we publish the English description. Thus, each post in the MTPReg register contains information about the Term, the Term description, the Code number and the appearance of the instrument.
The MTPReg code number is composed of two groups, exemplified by EUxxxxx-yyyyy. The first five digits (xxxxx) is a copy of the GMDN Code while the second group of five digits (yyyyy) is an MTP-Reg identification number for the make/model. The first number for each GMDN Code is always 00000.
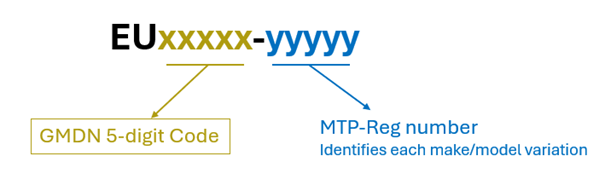
EU is the prefix we use when we create new MTPReg codes. This prefix separates a younger generation of codes from an older generation, with the prefix SE. The “EU codes” were introduced in 2020, when we first started to ask for two photographs of the medical device enclosed with the request. A real device example is shown below:

Using the two photos, together with other information supplied, enables us to perform a “quality control” of the code request by searching the Internet or even by contacting the producers to ask for additional information when we need it. In this way there are fewer mistakes made when we are setting the MTPReg codes.
Currently we have about 25,000 codes in the MTPReg register. We have started to slowly eliminate the older codes with a SE prefix to gently force our customers to apply for new EU codes. The term and term description will be the same as for the corresponding SE code, but the new codes will benefit greatly from the two photos included, which enables us to create more accurate codes for our customers, the Swedish public hospitals.
Annika Billberg
MTPReg Group
