
GMDN FOCUS – September 2023
GMDN FOCUS – September 2023 25 September 2023 Click on the link below to download

GMDN FOCUS – September 2023 25 September 2023 Click on the link below to download

GMDN Agency publishes landmark study on nomenclature mapping with EMDN 18 September 2023 Research highlights

Blog – A Single Globally Utilised Nomenclature is a Patient Safety Issue 11 September 2023

Blog – Maximising Stakeholder Engagement: A Multifaceted approach by the GMDN Agency 30 August 2023

GMDN FOCUS – Summer 2023 16 August 2023 Click on the link below to download

GUDID database updated to include GMDN Code 16 August 2023 The Global Medical Device Nomenclature

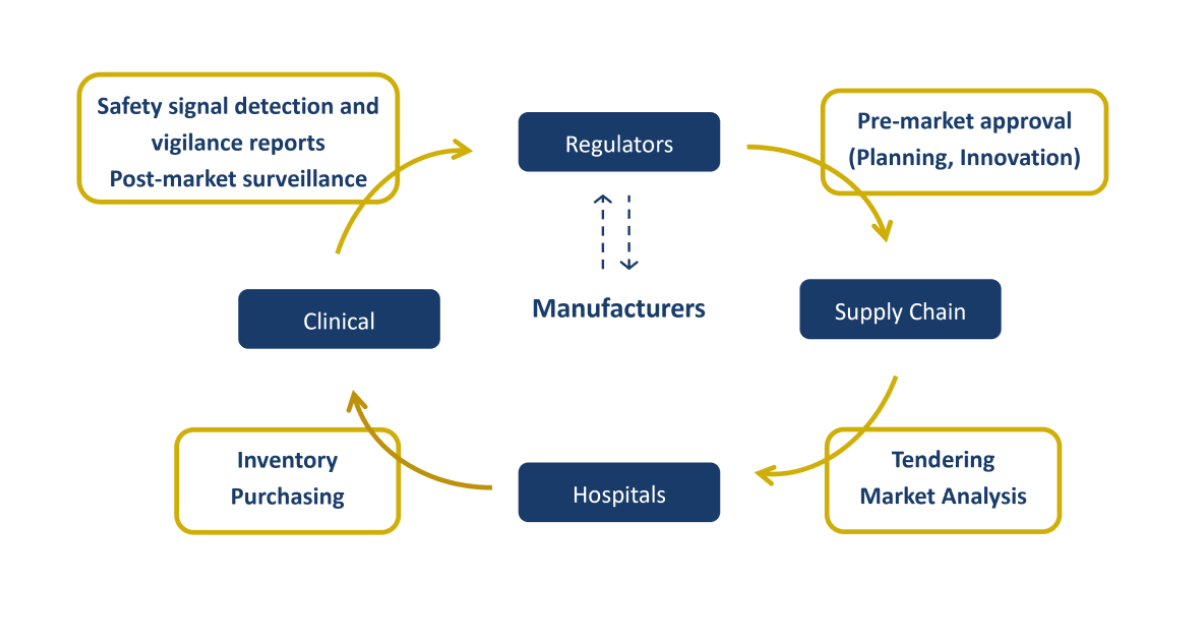
CEO Blog – How can the GMDN support post-market medical device surveillance and vigilance? 2

GMDN FOCUS – June 2023 28 June 2023 Click on the link below to download

The UK’s Medicines and Healthcare products Regulatory Agency (MHRA) embeds Global Medical Device Nomenclature (GMDN) in Public Access Registration Database