What We Do
What is GMDN
The Global Medical Device Nomenclature (GMDN) is the leading global standard for the naming, classification and categorisation of medical devices. Anyone can register for free as a member on the GMDN website to access and use any GMDN Term.
Our nomenclature provides Healthcare Professionals, Regulators, Manufacturers and others with a common language to communicate and share information.
GMDN enables safer and more effective patient care, fosters innovation and collaboration in the medical device industry, and supports global harmonisation of regulatory requirements.
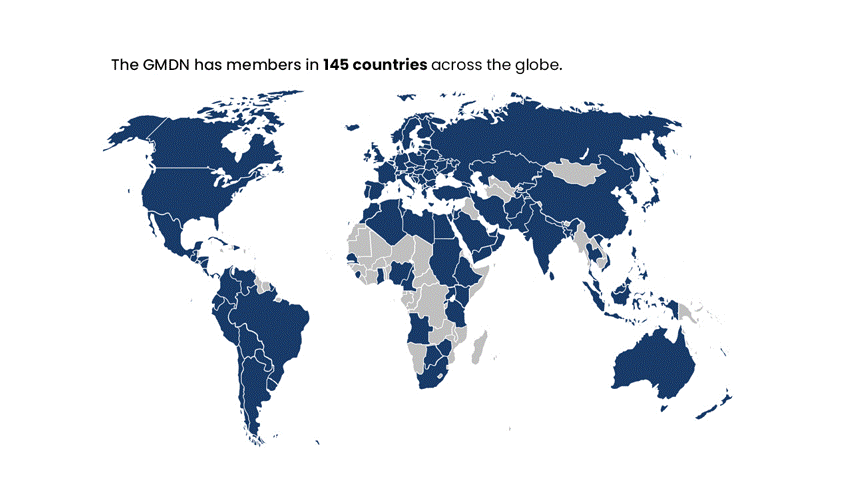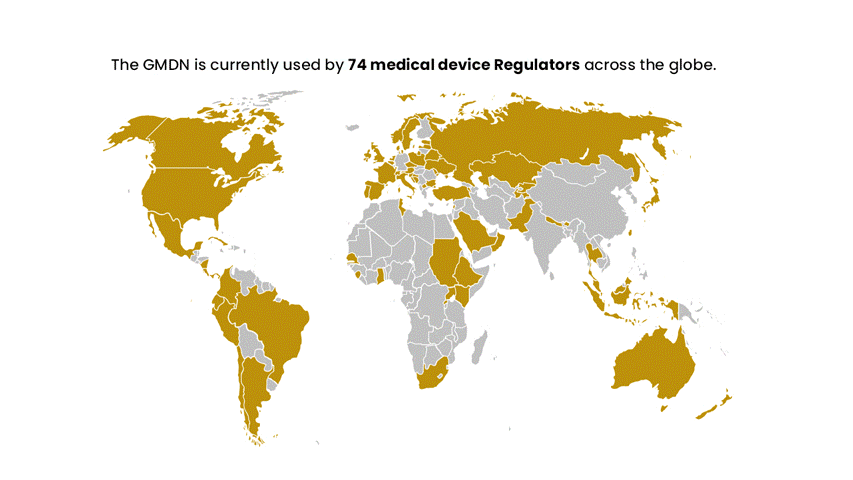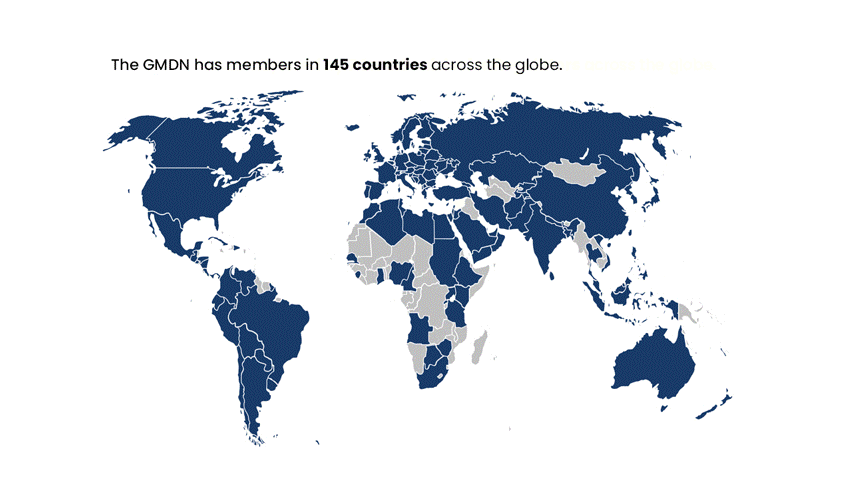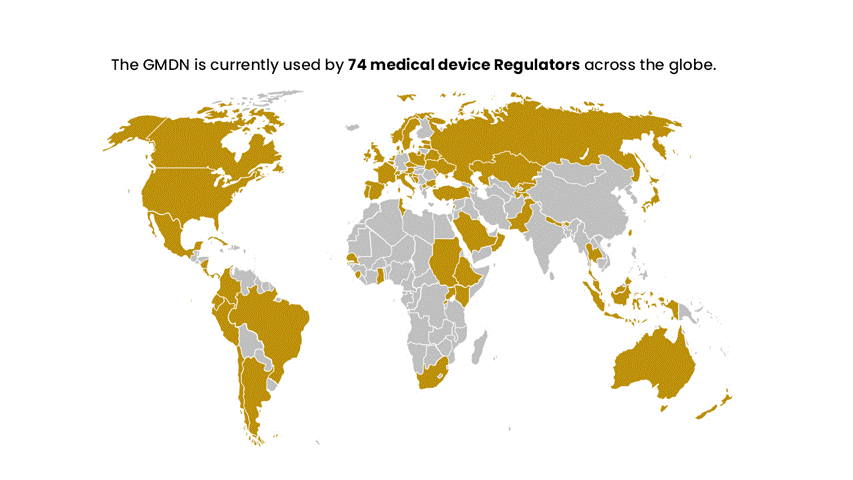
The GMDN is designed to be flexible and adaptable to accommodate new and emerging technologies, and it is continually updated to reflect changes in the medical device landscape. The system is used by Regulators in nearly 70 countries worldwide and has members in around 140 countries across the globe. It has become a critical component of the global regulatory infrastructure for medical devices.
The full GMDN is available for free to Regulators, Healthcare Providers and Academic Researchers.
What is the GMDN Agency?
Read more about our vision and objectives in our 5 year strategy.
You can download our latest annual report from the Charities Commission website.
-
Our Vision
To provide a single common language for all medical technology, and for it to be adopted by medical device regulators, manufacturers and other participants in healthcare systems worldwide.
-
Our Mission
To be the global leader in naming, describing and unambiguously identifying medical devices for the protection of patients
How we keep the GMDN updated?
The GMDN Agency employs a team of expert Term Developers who analyse new products and technologies in the MedTech industry to create concise and accurate descriptions. If a product is unique, a new GMDN Term is created, but if it’s a variant of an existing product, an amendment to the Term Name or Definition may be made. Occasionally, a review of GMDN Terms may be needed to determine if they reflect the current devices on the market.
The decision to amend or create a new Term is made in consultation with manufacturers, sometimes with regulators’ input, and is dependent on the complexity of the product and the impact of any changes on the GMDN Database.
The GMDN Agency aims to complete the processes as soon as possible to make the updated information available to manufacturers and regulators.
GMDN global use
In 1991 the first international workshop on medical device nomenclatures was held to create the GMDN. Initially started by the European Standards Organisations (CEN) and later supported by the Global Harmonisation Task Force (now the International Medical Device Regulators Forum – IMDRF) to help accelerate the harmonisation of medical device regulation globally.
The GMDN is recommended by the IMDRF and is now used by circa 70 national medical device regulators across the globe. The GMDN is managed by the GMDN Agency, a registered charity, which has a Board of Trustees representing regulators and industry.
The GMDN is currently used by around 70 medical device Regulators across the globe and has members in around 140 countries worldwide.